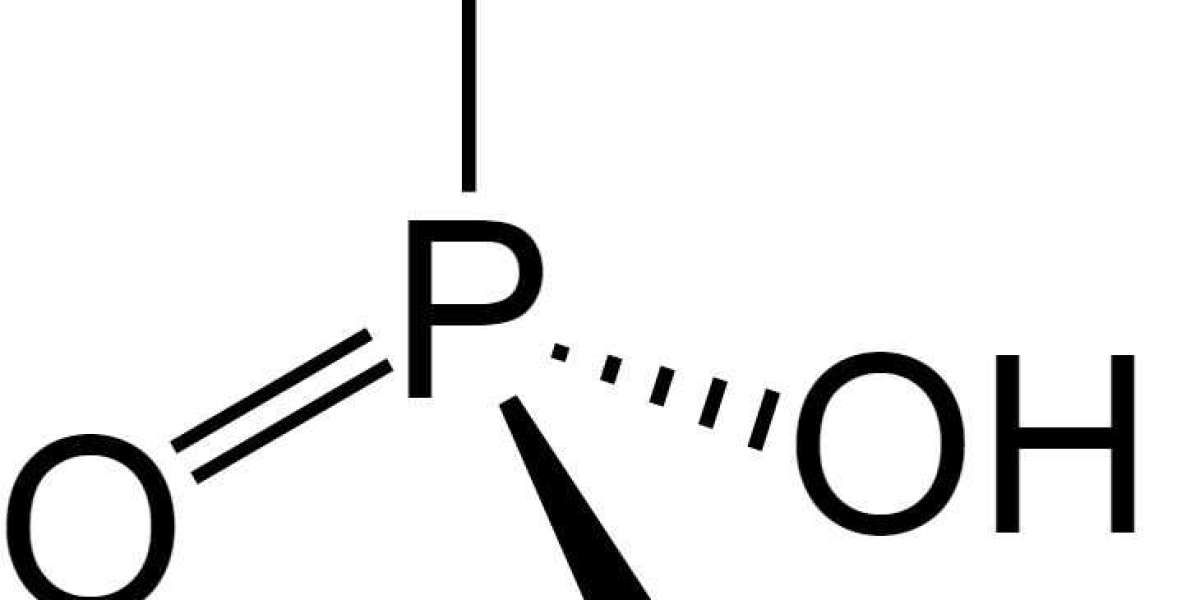
What is phosphoric acid?
Phosphoric acid is a mineral acid. This means it is an inorganic type of acid. These are derived from one or more inorganic compounds. In the case of phosphoric acid, they are derived from inorganic phosphate rocks. Acid of higher purity can also be made, by using white phosphorus. Other examples of mineral acids are sulfuric acid, hydrochloric acid, and nitric acid. When dissolved, mineral acids form hydrogen ions and conjugate base ions.
Its IUPAC name is orthophosphoric acid. IUPAC stands for International Union of Pure and Applied Chemistry. It is the universally-recognized authority on chemical nomenclature and terminology. This system allows them to establish unambiguous, consistent, and uniform nomenclature and terminology for specific scientific fields. Through this, confusion is avoided and identification of substances are simplified.
Phosphoric acid, in its pure form, is a crystalline solid, but it is easily dissolved into a viscous liquid. In this latter form, it is a colorless and odorless solution. When sufficiently diluted in water, it has a sour taste.
There are two methods used in the production of phosphoric acid. The difference between the two is the purity of the product produced.
The first method is the WET METHOD. It is the less expensive process of the two. Concentrated sulfuric acid (93%) is added to tricalcium phosphate rock. This is typically found in nature as the substance fluorapatite. The products of this reaction are phosphoric acid and calcium sulfate or gypsum, along with other impurities. At this stage, water is added so that gypsum and other insoluble materials are removed through filtration.
This is then purified through the evaporation of water. This removes fluoride to produce an animal-grade phosphoric acid, or further purified through solvent extraction and arsenic removal to produce food-grade phosphoric acid.
Generally, due to its economical nature, wet-process phosphoric acid is widely used in the commercial sector. It is typically used for the manufacture of fertilizers.
In other instances, nitric acid is used instead of sulfuric acid. This method co-produces calcium nitrate, which is another plant fertilizer. However, this method is very rarely used.
The second is the THERMAL METHOD. This process uses two key reactants: phosphorus and air. Phosphorus is sprayed into a furnace, with the air temperature at 1800 to 3000 K. The process usually employs moist air and involves the addition of steam. This allows a film of condensed polyphosphoric acids to be produced and maintained. This helps protect the stainless steam burner tower which is also externally water-cooled. The products from the burner tower head directly into a hydration tower. In here, the gaseous phosphorus oxide is absorbed in recycled phosphoric acid. The product is a purer food-grade acid with a concentration of 85%. This method is typically used in the food industry.
Phosphorous also creates other oxyacids. These include phosphorous acid (H3PO3) and hypophosphorous acid (H3PO2).